Discover the future of medical devices! By 2023, the market is expected to hit US$ 603 billion, growing at a 5% CAGR. Dive into the details here: reports.valuates.com/market-reports… #HealthcareGrowth #MedicalDevices


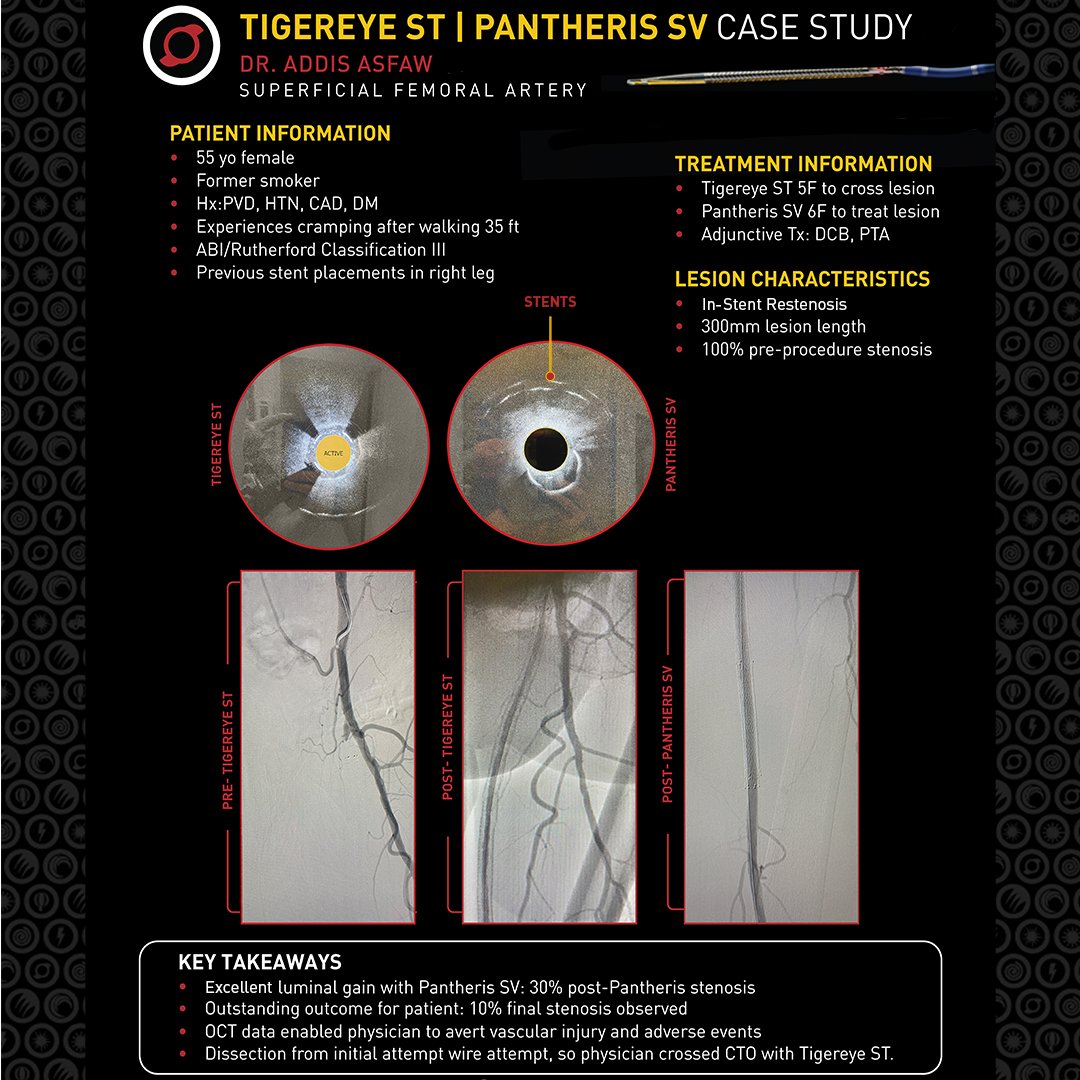
🥇First-time use of Avinger’s Tigereye ST & Pantheris SV leads to remarkable success in a difficult ISR CTO case! Dr. Asfaw achieved excellent results without additional stenting. A true testament to innovation in patient care! #FirstUse #MedicalDevices #HealthTech #PatientCare


Attention Medical Device Companies❗
Mark your calendars - May 26, 2024, is the deadline to transition from the European Medical Devices Regulation (MDD) to the new Medical Devices Directive (MDR). Get up to speed on what this means - watch our explainer video.
#medicaldevices

Exciting breakthrough in medical tech! Rani Therapeutics' new patent app #US20240139479 unveils a swallowable device that propels drugs directly into the GI tract wall, bypassing degradation. This could revolutionize treatment delivery! #HealthTech #Innovation #MedicalDevices


Day in the Life of a Regional Sales Manager Sales Meeting in Medical Device Sales
#medicaldevice s ales #medicaldevice s #medicaldevice #medicalsales #medicalsales college #sales #success #newtomedicaldevicesales #medicine #medtech

Locking Narrow Dynamic Compression Plate- 4.5/5.0mm K & Apple K and Apple #locking #orthopedic #orthopedic surgery #orthopedic s #orthopedic surgeon #surgery #orthopedic implants #LockingNarrowDynamicCompressionPlate #doctor #orthopaedics #medical #ortopedia #medical devices #hospital

Placa de compresión dinámica estrecha con bloqueo -4,5/5,0 mm K & Apple K y Apple #locking #orthopaedic #orthopedicsurgery #orthopaedic s #orthopedicsurgeon #surgery #orthopedicimplants #LockingNarrowDynamicCompressionPlate #doctor #orthopaedic s #medical #ortopedia #medical devices

comparison! comparison!! comparison!!! 😲 We are just better. #MedTechMarvels #HealthInnovation #MedicalDevices #HealthTech #MedDevice #MedTechTrends #MedicalEquipment #HealthTech Solutions #MedTechIndustry #MedicalInnovation #HealthTech News #MedDevice Innovations X

👋 Studing abroad?
Watch me post for more information!
#Translations #Traducciones
#Translator #Traductora
#RegulatoryAffairs #AsuntosRegulatorios
#MedicalDevices #DispositivosMédicos
#PersonalDocuments #DocumentaciónPersonal
#English_Spanish #Inglés_Español

'Looking to navigate the FDA's Pre-Submission Program for medical devices and IVDs? Our experienced US consulting team can guide you through this critical step toward FDA clearance.
Follow us at: ND Global
#FDA #MedicalDevices #IVD #RegulatoryCompliance '

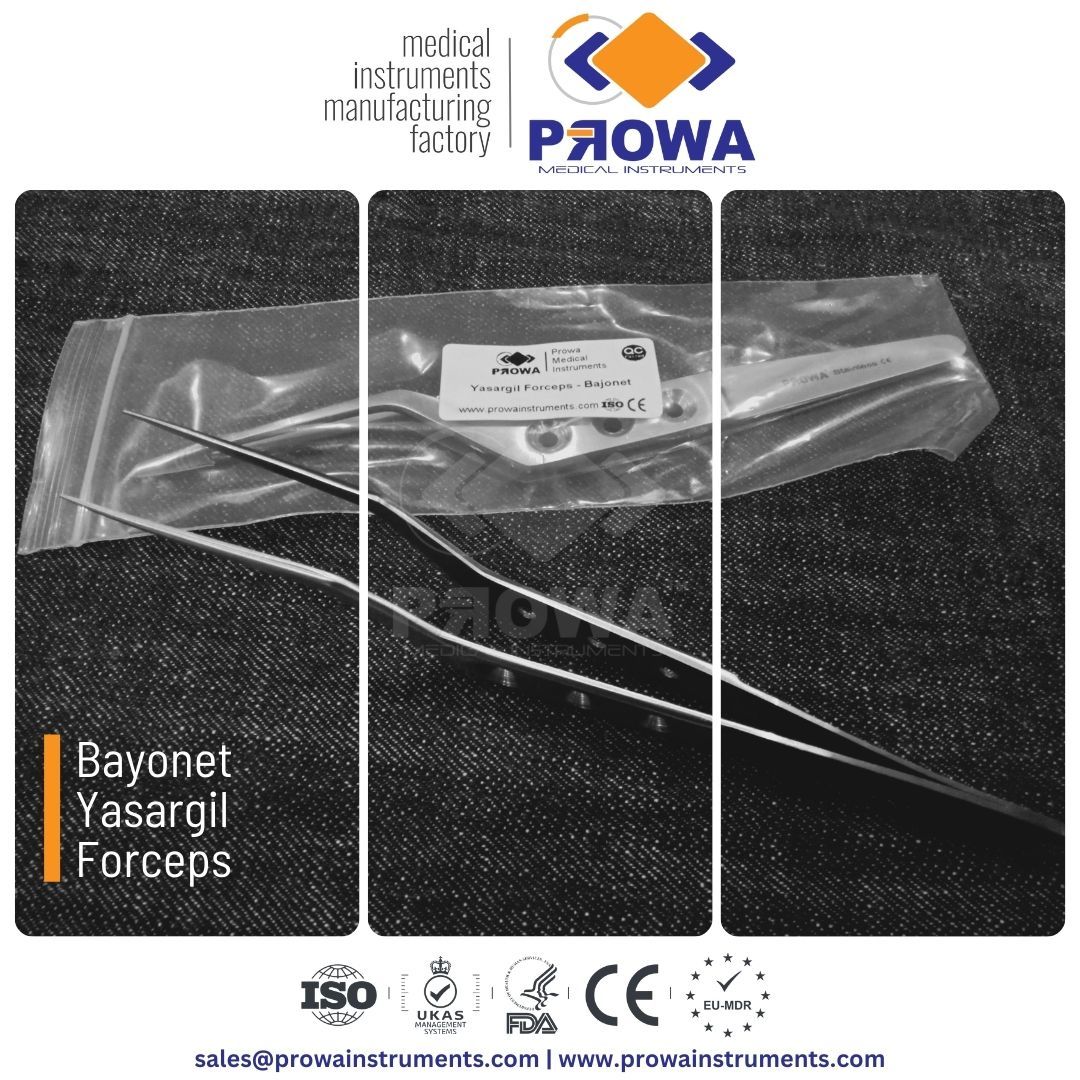
Bayonet Yasargil Forceps
.
.
.
#dental #dental factory #dental surgery #dental health #dental world #stainlesssteel #medicalinstruments #medicalequipments #health #medicaldevices #medicaldevicemanufacturing #surgical #surgical tech #surgical steel #surgical Instruments #madeinpakistan

#TIHE
🎉 Highlight photos from the amazing and fruitful TIHE, the biggest medical exhibition in Uzbekistan!🥳 #singclean #innvation #medicaldevices


Orveta.com
Premium Domain for sale ✅
Available at:
Dan.com and GoDaddy.com
#domainforsale #BrandableDomains #Health Care #Health Tips #premiumdomains #Medical #care #medicaldevices #Health #beauty #Medicine #Clinic #Startups #healthy